
Correct Mixing & Administration, enabling Subcutaneous (SC) Twice-a-Year Delivery
Fensolvi dosing—one 45 mg SC injection every 6 months
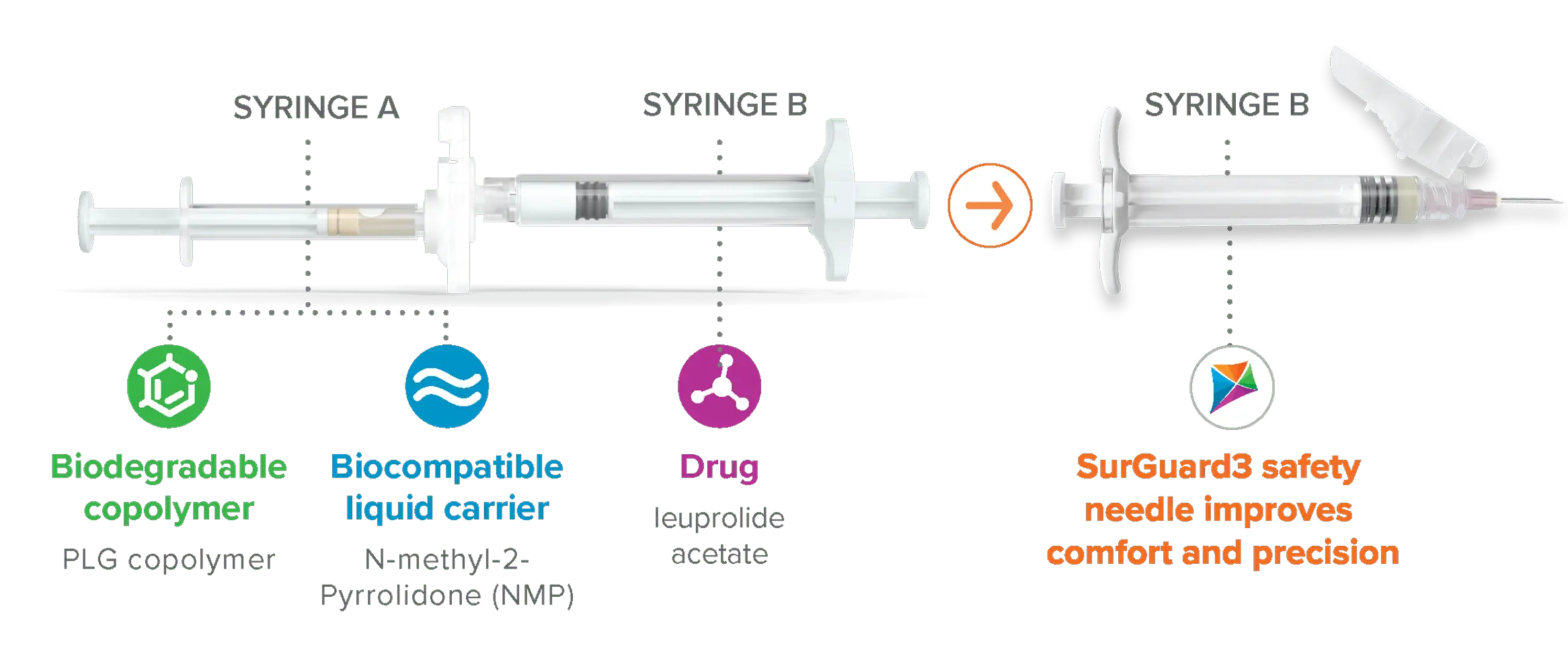
Fensolvi administration:
Good to Know: Key steps for the optimal patient treatment experience
Prepare
Room temperature
Allow the product to reach room temperature before using1
Mix
60 cycles
Thoroughly mix the product for 60 cycles1
Administer
90° angle
Inject slowly at a 90° angle into subcutaneous tissue—visually inspect to make sure all the drug has been injected before removing the needle at a 90° angle1
Fensolvi comes in a tray containing the pre-connected syringe system and a safety needle
Before mixing, prepare the Pre-connected Syringe System:
- Open the tray with gloved hands and place contents on a clean surface.
- Discard the desiccant pack and prepare the needle for attachment.
- Before mixing Fensolvi, first allow it to come to room temperature.
Not actual size
An overview of Fensolvi Preparation and Mixing
Preparing Fensolvi is a multi-step process using the materials provided and should only be done according to the detailed instructions in the Prescribing Information.

Storage & Packaging
Cold Storage
Store Fensolvi at:
- Store Fensolvi at 35.6–46.4 °F (2–8 °C)1
Room temperature storage— up to 8 weeks:
- Fensolvi may also be stored in its original packaging at room temperature 59–86 °F (15–30 °C) for up to 8 weeks prior to mixing and administration1
Packaging
Specifications:
- Fensolvi is prefilled and supplied in a sterile, preconnected syringe system that will need to be reconstituted
- The package dimensions are 10.875″ (w) x 3.2″ (h) x 1.4″ (d)
Contact us to schedule a live in-service training with your local Account Manager
Alternatively, a Virtual Training Session includes a live demonstration on mixing and administering Fensolvi. The clinical trainer will answer any questions you may have.
Select a date and time below to schedule virtual training.
Connect with Fensolvi
Connect with a Fensolvi Representative
By providing your information, you are giving Tolmar, Inc. and other parties working with us permission to communicate with you about Fensolvi or other products, services, and offers from Tolmar, Inc. If you don’t find the information useful, you can opt out at any time. We value your privacy and we encourage you to review our privacy policy for more information.
"*" indicates required fields
Fensolvi Starter Kits
We’re excited to offer Fensolvi starter kits for new patients who have been prescribed Fensolvi. Each kit offers helpful information for caregivers plus engaging activities for children to enjoy during their visits. Contents include a treatment brochure, coloring and iSpy sheets, coloring pencils, a book of jokes, and coping cards—all inside a handy drawstring bag. Order kits for your patients by calling your Tolmar representative or completing the form.
Contact us at 1-866-FENSOLVI (336-7658)
"*" indicates required fields
Contact us to schedule a mixing and administration demonstration
We will only use your submission to communicate with you about scheduling a mixing and administration training and to share associated materials. Please see our privacy policy for more information.
Contact us at 1-866-FENSOLVI (336-7658)
"*" indicates required fields
Sign up to receive clinical and support updates
Complete the form below to receive emails featuring key updates related to Fensolvi. By providing your information, you are giving Tolmar, Inc. and other parties working with us permission to communicate with you about Fensolvi® or other products, services, and offers from Tolmar, Inc. If you don’t find the information useful, you can opt out at any time. We value your privacy and we encourage you to review our privacy policy for more information.
Contact us at 1-866-FENSOLVI (336-7658)
"*" indicates required fields
IMPORTANT SAFETY INFORMATION
FENSOLVI® (leuprolide acetate) for injectable suspension is a gonadotropin releasing hormone (GnRH) agonist used to treat patients 2 years of age and older with central precocious puberty (CPP). CPP may be diagnosed when signs of sexual maturity begin to develop in girls under the age of 8 or in boys under the age of 9.
FENSOLVI is contraindicated in individuals with hypersensitivity to any drug that is in the same class as FENSOLVI, in individuals who are allergic to any of the ingredients in FENSOLVI, or in individuals who are pregnant. FENSOLVI may cause fetal harm when administered to a pregnant patient.
During the first few weeks of treatment, increases in gonadotropins and sex steroids above baseline may result in an increase in signs and symptoms of puberty including vaginal bleeding in girls.
Psychiatric events have been reported in patients taking GnRH agonists. Events include emotional lability, such as crying, irritability, impatience, anger, and aggression. Patients should be monitored for development or worsening of psychiatric symptoms.
Convulsions have been observed in patients treated with GnRH agonists with or without a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and in patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs.
Severe cutaneous adverse reactions (SCARs), including Stevens-Johnson syndrome/toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, and acute generalized exanthematous pustulosis, have occurred in patients receiving GnRH agonists. Monitor for and advise patients of the signs and symptoms of SCARs.
Pseudotumor Cerebri (Idiopathic Intracranial Hypertension) has been reported in pediatric patients treated with GnRH agonists. Patients should be monitored for headache, papilledema and blurred vision.
The most common adverse reactions seen with FENSOLVI were: injection site pain, nasopharyngitis, pyrexia, headache, cough, abdominal pain, injection site erythema, nausea, constipation, vomiting, upper respiratory tract infection, bronchospasm, productive cough and hot flush.
Please see Full Prescribing Information for FENSOLVI for additional important safety information.
To report suspected adverse reactions contact Tolmar at 1-844-4TOLMAR (486-5627) or the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
IMPORTANT SAFETY INFORMATION
FENSOLVI® (leuprolide acetate) for injectable suspension is a gonadotropin releasing hormone (GnRH) agonist used to treat patients 2 years of age and older with central precocious puberty (CPP). CPP may be diagnosed when signs of sexual maturity begin to develop in girls under the age of 8 or in boys under the age of 9.
FENSOLVI is contraindicated in individuals with hypersensitivity to any drug that is in the same class as FENSOLVI, in individuals who are allergic to any of the ingredients in FENSOLVI, or in individuals who are pregnant. FENSOLVI may cause fetal harm when administered to a pregnant patient.
During the first few weeks of treatment, increases in gonadotropins and sex steroids above baseline may result in an increase in signs and symptoms of puberty including vaginal bleeding in girls.
Psychiatric events have been reported in patients taking GnRH agonists. Events include emotional lability, such as crying, irritability, impatience, anger, and aggression. Patients should be monitored for development or worsening of psychiatric symptoms.
Convulsions have been observed in patients treated with GnRH agonists with or without a history of seizures, epilepsy, cerebrovascular disorders, central nervous system anomalies or tumors, and in patients on concomitant medications that have been associated with convulsions such as bupropion and SSRIs.
Severe cutaneous adverse reactions (SCARs), including Stevens-Johnson syndrome/toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, and acute generalized exanthematous pustulosis, have occurred in patients receiving GnRH agonists. Monitor for and advise patients of the signs and symptoms of SCARs.
Pseudotumor Cerebri (Idiopathic Intracranial Hypertension) has been reported in pediatric patients treated with GnRH agonists. Patients should be monitored for headache, papilledema and blurred vision.
The most common adverse reactions seen with FENSOLVI were: injection site pain, nasopharyngitis, pyrexia, headache, cough, abdominal pain, injection site erythema, nausea, constipation, vomiting, upper respiratory tract infection, bronchospasm, productive cough and hot flush.
Please see Full Prescribing Information for FENSOLVI for additional important safety information.
To report suspected adverse reactions contact Tolmar at 1-844-4TOLMAR (486-5627) or the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
References
Fensolvi (leuprolide acetate). Package insert. Fort Collins, CO: Tolmar, Inc.; 2025.
FENSOLVI TOTALSOLUTIONS COPAY PROGRAM TERMS AND CONDITIONS
The Fensolvi® Copay Assistance Program (“Program”) is valid ONLY for patients who are prescribed Fensolvi® and are reimbursed exclusively by commercial insurance. This Program is valid only in the United States; but, void where prohibited by law or by the patient’s health insurance provider. This Program is non-transferable, limited to one per person, and cannot be combined with any other coupon, free trial, discount, prescription savings card, or other offer. Restrictions or limits may apply.
Medicare, Medicaid, Tricare and other federal health care program beneficiaries may not participate in this Program. This Program also is neither available for cash paying patients nor where your commercial plan reimburses you for the entire cost of your prescription drug. Patients cannot seek reimbursement from health insurance or any third party for any part of the assistance received through this Program. The patient or his/her guardian is responsible for reporting the receipt of all benefits or reimbursement received under the Program to any insurer, health plan, or other third party, as may be required. This Program is not insurance and is not intended as a substitute for insurance.
With the Program, you pay as little as $5 of your co-pay or co-insurance for Fensolvi®, per prescription. The remainder of your co-pay or co-insurance is covered, up to two prescriptions per calendar year. The Program assists with the cost of Fensolvi only. It does not assist with the cost of other administrations, medicines, procedures or office visit fees.
Tolmar, Inc. (“Tolmar”) reserves the right to terminate, rescind, revoke, or modify this Program at any time without notice. This Program expires at the end of the current calendar year, at which time you must re-enroll. For complete information about the terms and conditions of this Program, including the limitations on use and the amount of assistance call 1 866-FENSOLVI (336-7658).
Program managed by Scripts Rx on behalf of Tolmar.
